Edward Wood, MD
Vitreoretinal Surgery Fellow
ARC/Beaumont
Following opening comments by Timothy Murray, MD, MBA, the neovascular AMD symposium kicked off an exciting series of symposiums. The section moderated by David Brown, MD, and David Williams, MD, MBA was divided into several talks given by 3-4 leaders in our field centering around a common theme, followed by a brief discussion. Here, we’ll discuss the data presented by maintaining this group structure and the ensuing discussion.
Group #1 – Brolucizumab, clinical trials, and the anti-VEGF drying effect
David Brown, MD, Pravin Dugel, MD, and Arshad Khanani, MD began the neovascular AMD symposium by presenting data from two Phase 3 trials (HAWK and HARRIER) using brolucizumab (RTH258), a novel anti-VEGF therapeutic developed by Novartis. Brolucizumab is a single-chain antibody fragment that is much smaller (at 26 kilodaltons (kDa)) than other available anti-VEGF therapeutics (when compared to ranibizumab’s 48 kDa and aflibercept’s 115 kDa). This allows brolucizumab to have more efficient tissue penetration and more rapid systemic clearance. HAWK (NCT02307682) and HARRIER (NCT02434382) are prospective, randomized, double-masked, 2-year non-inferiority studies evaluating the efficacy and safety of brolucizumab for the treatment of nAMD with the below study design. The trial are very similar, with HAWK randomizing patients (1:1:1) to brolucizumab 3mg, brolucizumab 6mg, or aflibercept 2mg, while HARRIER randomized patients (1:1) to brolucizumab 6mg or aflibercept 2mg. In the image, below, the icon of an eye represents a timepoint when disease activity was assesed (the first being at 16 weeks after identical loading phases between the agents).

David Brown, MD presented data on the predictive analysis of the 12-week dosing status at week 48 for patients receiving brolucizumab in the HAWK and HARRIER studies. Dr. Brown discussed that the retinal drying effect (re: subretinal fluid and sub-RPE fluid) of brolucizumab was superior to aflibercept at both week 16 and 48. When correlating this with real-world clinical practice with 71% of physicians responding to the ASRS PAT survey employing treat and extend based on a dry OCT, this data may translate into decreased treatment burden with superior retinal drying when compared to currently available therapeutics. Despite this superior drying effect, mean BCVA was similar between the groups.

Pravin Dugel, MD also presented data comparing the anatomical efficacy of brolucizumab versus aflibercept in wet AMD, but focused on the matched 16-week results from the HAWK and HARRIER studies. Week 16 is an important timepoint in these studies because the loading phase treatment regimen (q4 weeks x3) was equivalent. Dr. Dugel stated that while there was no difference in BCVA, patients receiving brolucizumab 6mg appreciated a statistically significant decrease in disease activity assessment (DAA), central subfield thickness (CST), and overall retinal fluid status at this important time point.
Arshad Khanani, MD presented data on extended primary and secondary outcome measures from the HAWK and HARRIER studies. He discussed that brolucizumab achieved its primary endpoint of non-inferiority in BCVA change versus aflibercept at week 48 with comparable safety. He also discussed that 57% (HAWK) and 52% (HARRIER) of patients treated with brolucizumab 6mg were maintained on a q12 week interval after the initial loading phase up to week 48.
Group #2 – Anti-VEGF treatment protocols and outcomes in the real world vs. clinical trials
Peter Kertes, MD, FRCS(C), Andrew Moshfeghi, MD, MBA, David Eichenbaum, MD, and Thomas Ciulla, MD, MBA presented data analyzing the impact of treatment protocols and number of injections on outcomes in wet AMD.
Peter Kertes, MD, FRCS(C) discussed the 1-year results of the Canadian Treat-and-Extend Analysis Trial (CANTREAT) with ranibizumab in patients with wet AMD. This was an investigator-initiated trial with 27 participating groups throughout Canada comparing treat and extend (T&E) to monthly dosing regimens for wet AMD using ranibizumab, with primary outcome measures of mean change in BCVA from baseline to month 12 and number of injections required. The T&E regimen itself was relatively aggressive given that patients were treated until no further improvement in OCT or BCVA occurred (disease stability). Patients were allowed two failed extension intervals before returning to fixed dosing. Using this protocol, it appeared that T&E resulted in significantly higher BCVA along with fewer required injections when compared to monthly dosing.

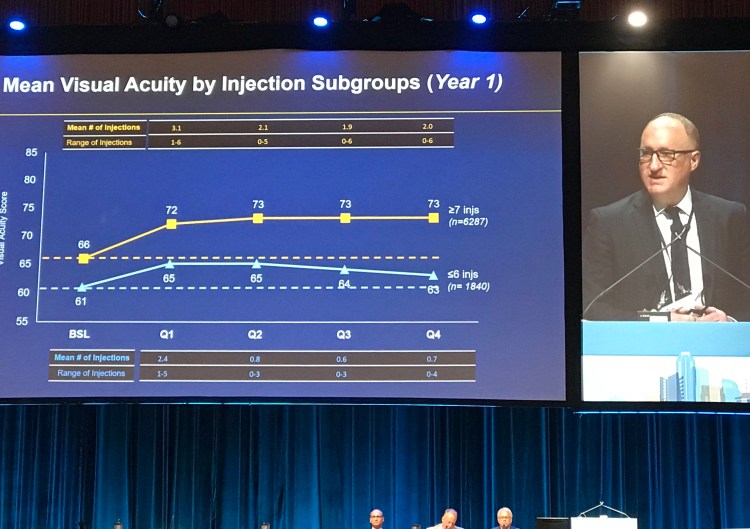
Andrew Moshfeghi, MD, MBA discussed outcomes of anti-VEGF therapy in routine clinical practice utilizing data from the VESTRUM HEALTH database. Patients receiving intravitreal anti-VEGF therapy between 2012 and 2015 were included, with analysis focused on two subgroups: (a) 6 or fewer injections per year and (b) 7 or more injections per year. Dr. Moshfeghi and colleagues found that the mean BCVA improvement was superior in those receiving 7 or more injections per year. While there was a trend towards more injections received in the first year of analysis, if patients had received 7 or more injections in the first year of analysis and the treatment regimen was then relaxed, patients did tend to lose vision. Patients receiving 6 or fewer injections in the first year who were then treated more aggressively in the second year or analysis did not tend to gain more vision in the second year, indicating that vision loss in wet AMD is difficult to recover. As opposed to the CANTREAT study, these results were consistent with results found in many randomized clinical trials suggesting that more injections resulted in improved visual outcomes.
David Eichenbaum, MD discussed the percentage of patients maintained on quarterly anti-VEGF dosing for the treatment of wet AMD across 6 key clinical trials (PIER, EXCITE, CABERNET, VIEW 1 and 2, HARBOR). He found that approximately 80% of patients with wet AMD maintained or gained vision on quarterly (q12 week) or extended dosing interval. He concluded his talk by suggesting that regular monitoring may help identify those who would respond well to less frequent dosing. and thus potentially decrease the burden of treatment.

David Williams, MD, MBA presented data on a real-world analysis of 49,485 eyes of visual acuity outcomes and anti-VEGF therapy intensity in wet AMD patients. Patients in the real world with wet AMD may differ significantly from those in RCTs, especially in regards to features that would exclude them from RCT inclusion such as >50% fibrotic component of lesion, >50% hemorrhagic component of lesion, significant subfoveal hemorrhage, central photoreceptor or RPE atrophy, and poor baseline BCVA (<20/200). Due to these features and data suggesting potential undertreatment of disease, Dr. Williams concluded by stating that real world wet AMD outcomes in the US are worse than those observed in RCTs. While number of injections does tend to correlate with BCVA outcomes, there appears to be a “ceiling effect” of treatment at 10 injections.

Group #3 – Anti-VEGF fellow eye effect, anatomical considerations, and reduced fluence PDT
Robert Avery, MD presented data on the fellow eye effect of anti-VEGF agents in the treatment of wet AMD with focus on the incidence of developing a CNVM in the fellow eye. Dr. Avery and colleges first reported the fellow eye effect of bevacizumab in the setting of proliferative diabetic retinopathy (with regression of fellow eye NVD), but has also been observed in diabetic macular edema and retinopathy of prematurity at minimal doses (as low as 1/200th of typical dose has been observed). Bevacizumab has a longer half than other anti-VEGF agents with 40-60x systemic exposure. Dr. Avery performed a meta-analysis of clinical trials employing anti-VEGF agents for the treatment of wet AMD disclosing a statistically significant effect in reducing the incidence of fellow eye CNVM. The discussion ensued regarding the incidence of systemic side-effects, and there was general consensus that patients at very high risk for systemic ATEs, and especially those who have recently experienced an ATE, should undergo consideration for alternative agents to bevacizumab.

Glenn Yiu, MD, PhD presented compelling data based on his insightful anatomical hypothesis that the cilioretinal artery may be protective in the setting of advanced AMD based on its hemodynamic support of involved retinal structures. Dr. Yiu showed that eyes with cilioretinal arteries did indeed have decreased AMD severity as well as lower rates of CNVM. However, there was no difference in the prevalence of GA. This interesting talk provided interesting food for thought regarding the potential retinal vascular contribution to AMD severity.

Colin Tan, MBBS, MMed (Ophth), FRCSEd (Ophth) discussed a study comparing the outcomes of standard and reduced duration photodynamic therapy (PDT) for the treatment of polypoidal choroidal vasculopathy (PCV). Overall, he showed that there was no statistically significant difference in fluid reduction, BCVA, or polyp closure with standard and reduced duration PDT. This reduced duration protocol did seem to result in less choroidal thinning, These findings join a trend of innovations within PDT by reducing fluence, dose, and now duration.

Group #4 – Personalized medicine, the impact of syringe and technique on post-injection inflammation, prophylaxis of fellow-eye CNVM, and endophthalmitis rates with bilateral injections
Anne Hanneken, MD presented data on behalf of Paul Tornambe, MD regarding the potential utility of OCT angiography (OCTA) in monitoring response to therapy with different anti-VEGF agents. It is known that reperfusion of the CNVM develops before the occurrence SRF in the setting of disease recurrence– and that anti-VEGF therapy may only partially hit the CNVM. It is also known that the greatest drug effect is seen within roughly a week. Based on these principles, Dr. Hanneken presented dynamic (weekly) OCTA imaging on a single patient of Dr. Tornambe who received bevacizumab, aflibercept, and ranibizumab for wet AMD. They found a differential response to the lesion size with different agents at one week, with the least robust response occurring in this patient with bevacizumab. While these results alone in this single patient do not provide support for performing weekly OCT imaging, they do promote intriguing considerations regarding the future of personalized medicine and how OCTA may play a role.

Gustavo Melo, MD, PhD presented data regarding inflammation after aflibercept injection in association with the SR brand of syringe that releases silicone oil droplets. Their group experienced a series of cases of inflammation after aflibercept injection that were treated with topical steroids alone. Dr. Melo and colleagues found that the incidence of inflammation was associated with the SR (silicone) syringe as well as a per-injection technique of ‘flicking’ the syringe. They concluded that agitation of this typical syringe increases the formation of silicone-associated aggregates, and they did observe silicone oil bubbles in these eyes. The discussion involved using repeat aflibercept therapy after experiencing a case of inflammation, and Dr. Melo stated that he tends to continue with aflibercept given he hasn’t experienced repeat inflammation. Furthermore, it may be beneficial to not have syringes sit for a significant period of time between drawing up the medicine and injecting.

Maziar Lalezary, MD discussed a prospective controlled clinical trial (PREVENT) analyzing the potential prophylactic effect of ranibizumab in preventing the development of fellow eye CNVM. This study was prompted by the observation that conversion to wet AMD in fellow eyes occurs 17-35% within 2 years. Dr. Lalezary and colleagues hypothesized that intermittent ranibizumab therapy in eyes with non-neovascular AMD would decrease the conversion rate to wet AMD. In the interim analysis of this study implementing anti-VEGF therapy quarterly in such eyes, there was no statistically significant effect in reducing the occurrence of wet AMD.

Durga Borkar, MD concluding the symposium by presenting the Mid-Atlantic Retina / Wills Eye Hospital experience with bilateral same day intravitreal anti-VEGF injections regarding rates of endophthalmitis. Dr. Borkar discussed that most patients were receiving ranibizumab therapy for wet AMD, and that roughly 20-30% of patients (between 2012-2017) required bilateral injections. In this series, no cases of bilateral endophthalmitis occurred, suggesting that this is a safe approach.
